Abstract
Introduction
Tyrosine kinase inhibitor (TKI) therapy has dramatically improved the prognosis of CML, with life expectancy now approaching that of the general population. TKIs are, however, associated with impaired quality of life, toxicity and financial burden to the patient and economy. Treatment free remission (TFR) is achievable in approximately half of patients who attain a sustained deep molecular response (DMR), however, it is not yet fully elucidated how best to predict candidates for successful TFR attempt. It is even harder to predict which patients will achieve a sustained DMR for 2 years or longer, which is a pre-requisite for TKI discontinuation. If it were possible to identify the patients who will not achieve a sustained DMR with Imatinib, a TKI switch could be considered earlier in order to make them a candidate for TFR attempt.
Aims
We aimed to identify disease characteristics and molecular responses that can predict future achievement of Stable MR4.5 (defined as a reduction in BCR/ABL1 transcripts of 4.5 logs or deeper on repeated testing for 2 consecutive years) with frontline Imatinib.
Patients and Methods
We collected data on pre-TKI variables (baseline disease characteristics), post-TKI variables (molecular response at various timepoints) and outcomes in patients commencing frontline Imatinib in our institution from 1999 to 2014 (n=593).
Statistical analysis was performed using EZR software. Univariate analysis was performed by cumulative incidence method considering competing events and Gray test. Cut-offs for continuous variables were determined by recursive partitioning (rpart). Multivariable analysis was performed using Fine-Gray model.
Results
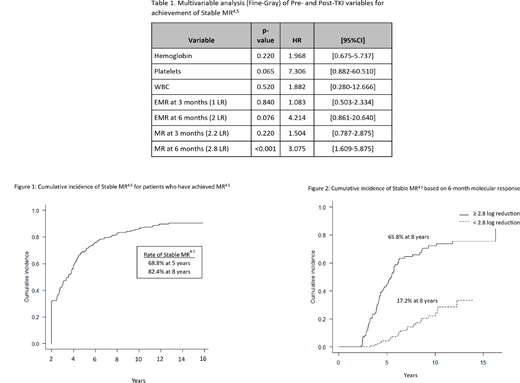
With 8.9 years of median follow-up duration, the overall survival was 96.9% at 8 years. The median time to MR4.5 was 8.8 years. The rate of MR4.5 was 39.7% at 5 years and 48.3% at 8 years. The rate of Stable MR4.5 was 25.6% at 8 years. In the subset of patients achieving MR4.5, over 80% subsequently achieved Stable MR4.5 (82.4% at 8 years) (Fig. 1). The median time from achievement of first MR4.5 to Stable MR4.5 was 3.5 years.
Univariate analyses of baseline variables (age, gender, disease phase, additional cytogenetic abnormalities and baseline blood counts) were performed, using rpart method to determine cut-offs for blood counts as follows: white cell count (WBC) ≥218x109/L, blast percentage ≥4%, hemoglobin (Hb) ≥88.5g/L and platelets ≥176x109/L. The only statistically significant pre-TKI variables on these analyses were WBC, blast percentage, Hb and platelet count.
Univariate analyses of the following post-TKI variables were also performed: molecular response at 3, 6 and 12 months, time to complete cytogenetic response, major molecular response and MR4.5.Early molecular responses of ≥1 log reduction in transcripts at 3 months, ≥2 logs at 6 months and ≥3 logs at 12 months were tested. The following cut-offs for molecular response, as determined by rpart method, were also tested: ≥2.2 log reduction at 3 months, ≥2.8 logs at 6 months and ≥3 logs at 12 months.Univariate analysis showed statistical significance (p<0.0001) for all the post-TKI variables tested.
Multivariable analyses of baseline blood counts and molecular response at 3 and 6 months were performed. The only variable that remained statistically significant was molecular response at 6 months using a cut-off of ≥2.8 log reduction in transcripts (HR 3.1, p<0.001) (Table 1). 44.4% of patients achieved ≥2.8 log reduction in transcripts at 6 months, with a rate of Stable MR4.5 at 8 years of 65.8%, compared to 17.2% for those with <2.8 log reduction at 6 months (Fig. 2).
Conclusions
In patients who achieved MR4.5, over 80% subsequently achieved Stable MR4.5, making them eligible for TKI discontinuation. In multivariable analysis, molecular response at 6 months was the only predictor for subsequent achievement of Stable MR4.5. Based on this data, a patient at high-risk of failing to attain Stable MR4.5 with Imatinib therapy can be identified if they fail to achieve a 2.8 log reduction or deeper within 6 months of Imatinib therapy. If a patient interested in TFR has a molecular response at 6 months of less than a 2.8 log reduction, then a switch in therapy to a second generation TKI may be considered. The optimal 6 month response to predict future Stable MR4.5 remains unclear, but our data suggest that a cut-off in transcripts of ≥ 2.8 log reduction may be a better predictor of future Stable MR4.5.
Lipton:ARIAD: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Kim:BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; Paladin: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal